What is a Piper diagram for water chemistry analysis and how to create one?
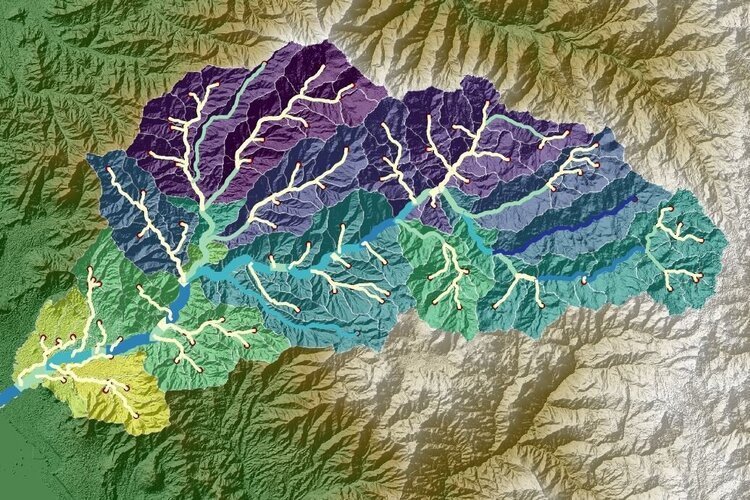
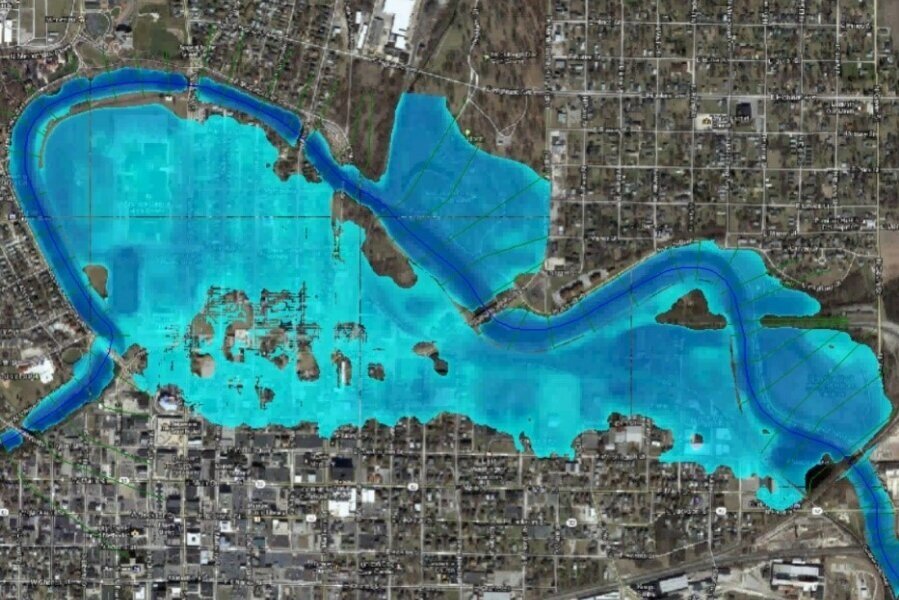
/In 1994, Arthur M. Piper, proposed an effective graphic procedure to segregate relevant analytical data to understand the sources of the dissolved constituents in water. This procedure was born under the statement that most natural waters contain cations and anions in chemical equilibrium. It is assumed that the most abundant cations are two “alkaline earths” calcium (Ca) and magnesium (Mg) and one “Alkali” sodium (Na). The most common anions are one “weak acid” bicarbonate (HCO3) and two “strong acids” sulphate (SO4) and chloride (Cl). Less common anion and cation-constituents are summed with the major three anions and cations as shown in the following table:
Read More